Study timing
Study timing displays the time elapsed between the acquisition of data and the presentation of data. The total transfer time for these processes must not exceed 15 minutes.
• The acquisition of data refers to when a study is sent from the modality.
• The presentation of data refers to when the full study is available for display on a diagnostic workstation.
The study timing tab includes three main sections:
• Study loading times
• Processing times
• Studies sent as copies

|
Note: This is a paid feature and is not enabled by default. If you are interested in this feature, please contact DH Healthcare GmbH to enable it.
|
Overview of the study timing tab
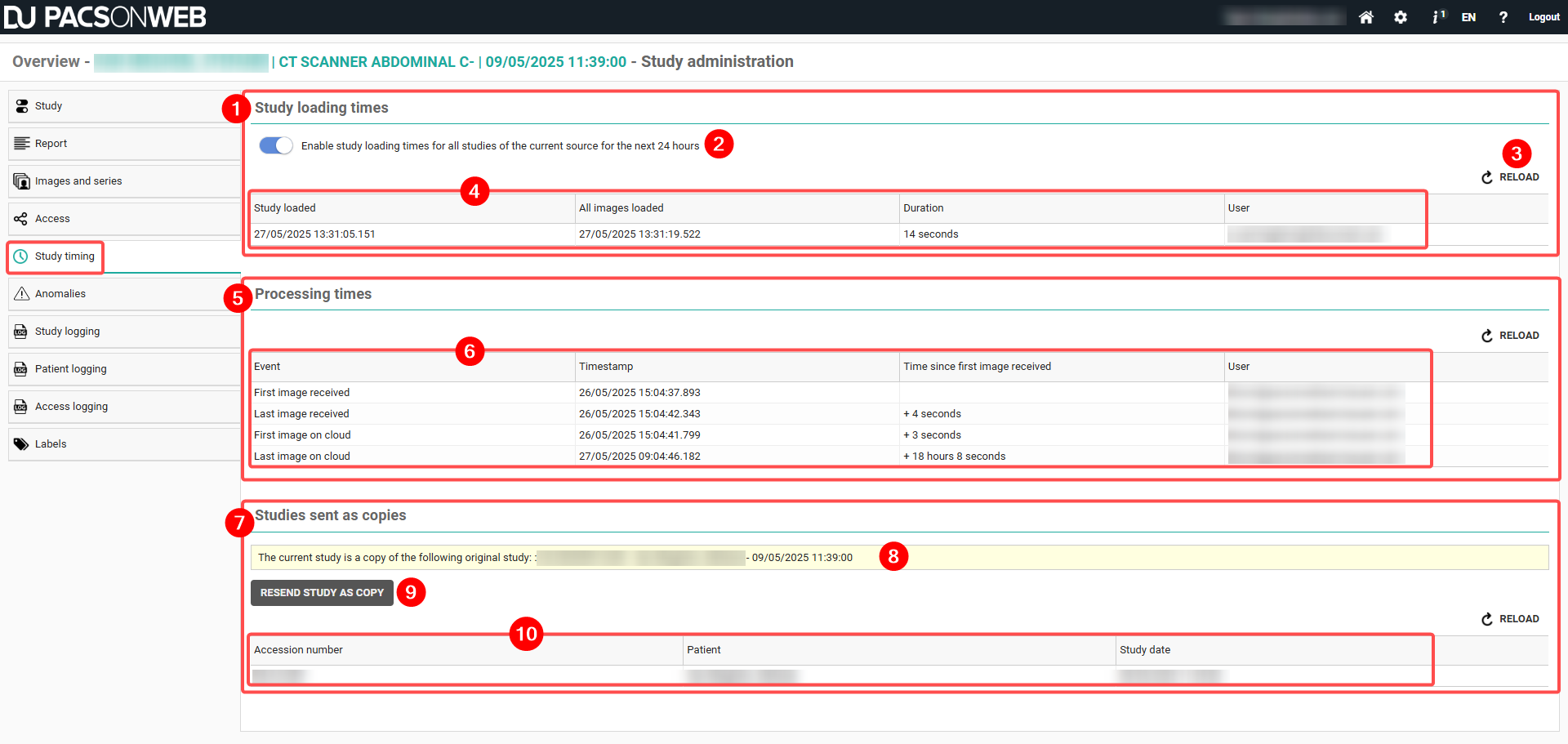
Number | Designation | Description |
|---|---|---|
1 | Study loading times | A section of the Study timing tab that displays timestamps and durations for the time it took for the study and all associated images to load. |
2 | Study loading times switch | A switch that when activated, enables study loading times for all studies of the current source for the next 24 hours. You can manually disable the switch before the 24-hour period. This switch is disabled by default. |
3 | Reload | A button that refreshes the information displayed in the table. Study loading times, processing times, and Studies sent as copies each have a respective reload button. |
4 | Study loading table | A table that displays relevant information about the study loading times, including: • The date and time the study was loaded • The date and time all images were loaded • The time it took to load the study • The user who loaded the study |
5 | Processing times | A section of the Study timing tab that displays information related to the processing time of the study. |
6 | Processing times table | A table that displays relevant information about the processing times: • Event • Timestamp • Time since first image was received • The user of the respective study |
7 | Studies sent as copies | A section of the Study timing tab that displays the studies that have been sent again, including the originating study if it was a study that was sent again. |
8 | Studies sent as copies message | A message is displayed if you open a study that was sent again as a copy. Studies sent again as copies are identified by an Accession number starting with RS-. Clicking this message will refresh the current page and display the original study. |
9 | Resend button | A button that triggers a new flow of processing measurements for the new study. |
10 | Studies sent as copies table | A table that displays relevant study information, including: • Accession number • Patient • Study date |
To enable the study timing feature
To enable this feature, you first need to enable the following switches:
1. Always load full image data for window level
◦ Go to: User settings > Preferences > Viewer > Viewer settings
◦ Enable: Always load full image data for window level
2. Enable study loading times for all studies of the current source for the next 24 hours
◦ Go to: The desired study > click the study administration icon > navigate to Study timing > Study loading times
◦ Enable: Enable study loading times for all studies of the current source for the next 24 hours
To create a measurement for a particular study after this feature is enabled:
1. Open the desired study in the viewer.
2. Select the image series All series and drag it into a viewport.
3. Wait for all images to load into the viewer. Successfully loaded images are indicated by a green progress bar below the image thumbnail.
4. Navigate to Study administration > Study timing tab.
The information about the study's loading and processing times will be displayed here.
Study loading times
This section of the Study timing tab displays timestamps and durations for the time it took for the study and all associated images to load and be displayed in the viewer.
• Measurements only take effect when 16-bit images are loaded.
• These measurements are triggered when the switch Enable study loading times for all studies of the current source for the next 24 hours is enabled.
• You can manually disable this feature by toggling the switch button to off.
◦ Once disabled, no new measurements for study loading will be triggered. The system does not record the times of any new studies loaded into the viewer.
◦ Past measurements that were executed while the feature was enabled will remain in the table.
The table for Study Loading Times will contain the following information:
• The date and time the study was loaded into the viewer.
• The date and time that all images in the specific series were fully loaded into the viewer.
• The duration it took to display all images.
• The user who displayed the study.
Processing times
This section of the Study timing tab displays the time required for processing the studies from the first image received from the modality to the last image available on the cloud.
• The processing times table is empty by default.
• An empty table displays the following message: No processing times are available for this study.
• When information is available, the following is displayed:
◦ Event: Predefined values indicating four measurements, listed chronologically from the oldest to the newest event.
◦ Timestamp: Date and time when the images matching the event were received.
◦ Time since first image received: The time elapsed between the first image received and each subsequent step. This clearly indicates the time taken for the DICOM processor to fully process the images and make them available in the cloud.
◦ User: The user who triggered the event.
Measurements are triggered based on a specific DICOM tag, 'EnableStudyTiming'. This tag is added to studies that are sent again using the Resend study as copy button in the Studies sent as copies section.
Studies sent as copies
This section of the Study timing tab displays studies that have been sent again and allows you to resend a study that is already available, triggering the processing times. This feature helps you periodically check the smoothness of the process.
• If there is not originating study, the panel will display this message: The current study is not a copy, so there is no original study.
• The studies sent as copies table is empty by default. No studies sent as copies are available for this study.
• All studies sent as copies will be listed in the table, displaying the following information:
◦ Accession number: Always starts with "RS-"
◦ Patient: Pseudonymized (Last name = Anonymous, First name = 6 characters)
◦ Study date: The date/time when the resend was triggered
If you open a study that was sent again as a copy, a message will be displayed indicating that the current study is a copy of the original study with the study details. This message is a link that will refresh the current page and display the original study.
Resend study as copy
This button triggers a new flow of processing measurements for the new study.
• The button will be temporarily disabled while the task is in progress.
• Once the process is complete, you can trigger another resend if needed.
If the button remains disabled, please contact your Global Administrator to ensure a QR server is configured with the option 'Use for Resend as copy'. If this option is not enabled, the button will remain grayed out.